The chemical rocket experiment kit is a kit with which you can carry out the chemical rocket experiment. The experiment consists of creating a small rocket propelled by CO2. CO2 is formed by the reaction between citric acid and potassium bicarbonate.
CONTENTS OF THE CHEMICAL ROCKET KIT
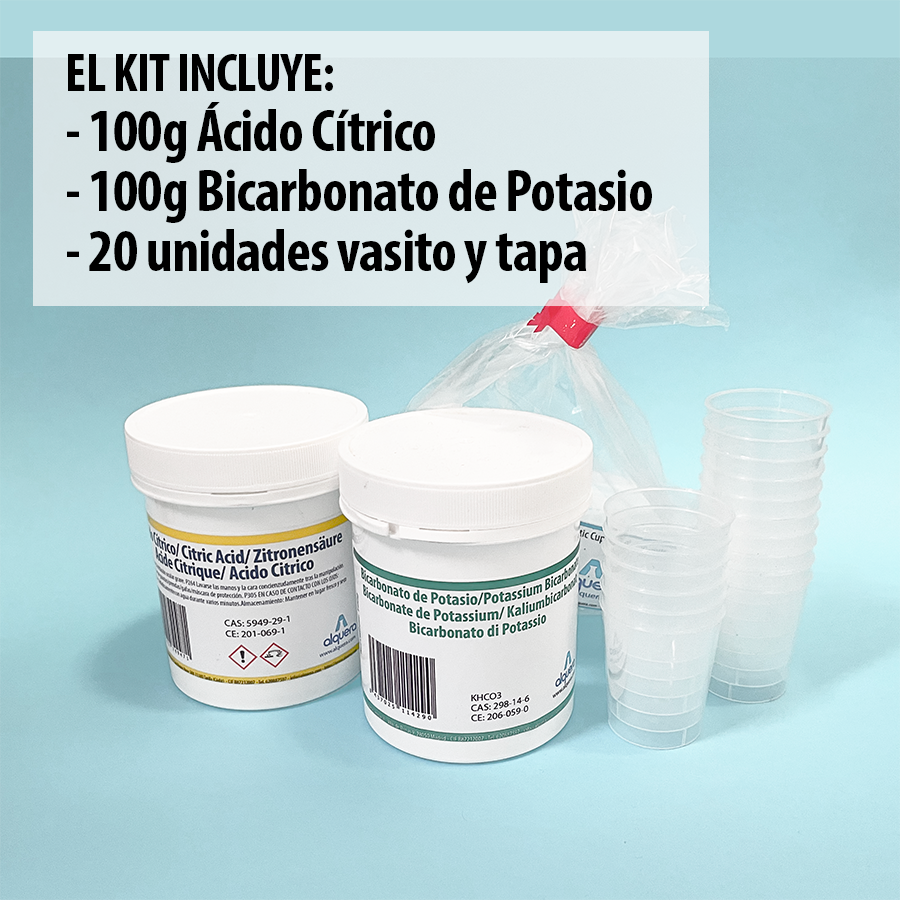
The kit is intended for 10-20 uses/person and contains:
- 100 grams citric acid
- 100 grams potassium bicarbonate
- 20 beakers of 25ml with 20 lids for airtight sealing
Kit does not contain: cellulose paper (toilet paper) and spoon
DETAILED STEP-BY-STEP
We show you the detailed step-by-step video and you can also follow the steps described below.
- Take a 25 ml glass and fill it halfway with water.
- Rotate the glass to make sure that the water covers all the sides of the glass.
- Place a piece of toilet paper in the glass over the water.
- Add half a tablespoon of citric acid on the paper.
- Add half a tablespoon of potassium bicarbonate to the paper.
- Cover the beaker tightly with a lid and place it on a flat surface.
- Take the glass with both hands and turn it upside down.
- Observe how the rocket is propelled upwards due to the pressure generated by the chemical reaction.
It is important to note that this experiment is only a home demonstration and should not be attempted at home without adult supervision. In addition, caution is advised when handling citric acid and potassium bicarbonate, as they can irritate the skin and eyes.
WHAT YOU LEARN FROM THIS EXPERIMENT
- How to make a homemade rocket experiment using common materials
- The basics of the chemical reaction between acid and base
- How pressure is generated and used to power a rocket
TECHNICAL EXPLANATION
The reaction between citric acid and potassium bicarbonate is a neutralisation reaction, in which a chemical reaction takes place between an acid and a base to form a compound called salts and water. The chemical reaction is as follows:
C6H8O7 (aq) + KHCO3 (aq) → K3C6H5O7 (aq) + H2O (l) + CO2 (g)
Where C6H8O7 is citric acid, KHCO3 is potassium bicarbonate, K3C6H5O7 is the citric salt formed in the reaction and H2O is water. CO2 is carbon dioxide, which is released in the reaction as a gas.
In this reaction, citric acid and potassium bicarbonate combine to form a salt called potassium citrate, as well as water and carbon dioxide. The reaction is exothermic, which means that heat is released during the chemical reaction.
The reaction between citric acid and potassium bicarbonate is a common reaction in household chemistry and is used to make a variety of products, such as mints, cleaning tablets and household cleaning products.
TYPE OF REACTION
The reaction between citric acid and potassium bicarbonate is an acid-base reaction. See more acid-base reactions.

Reviews
There are no reviews yet.